Lipase, a type of hydrolytic enzyme, has been widely used in organic synthesis since the 1980s. The successful integration of lipases into the asymmetric synthesis of optically pure organic compounds is becoming even more important in terms of operational safety and simplicity, efficiency, and reduced environmental impact.
By combining classical kinetic resolution with cutting-edge science using transition metals, functional inorganic materials, and computational chemistry, we have expanded the applications of lipases, resulting in innovations that can meet the above requirements. Examples include dynamic kinetic resolution (DKR), which quantitatively converts racemates into optically pure enantiomers, and domino-type molecular transformations through DKR acylation of alcohols followed by intramolecular cyclization reactions.[1] The key to successful DKR is to develop racemization catalysts that can coexist with lipases. For example, we have developed our original catalyst VMPS4, in which oxovanadium species are immobilized on the pore surface of mesoporous silica, and applied it to DKR of various secondary and tertiary alcohols. In combination with a Ru catalyst, DKR of axially chiral biaryl compounds was also achieved.[2] As further developments of DKR, I would like to introduce the following three recent achievements of our group:
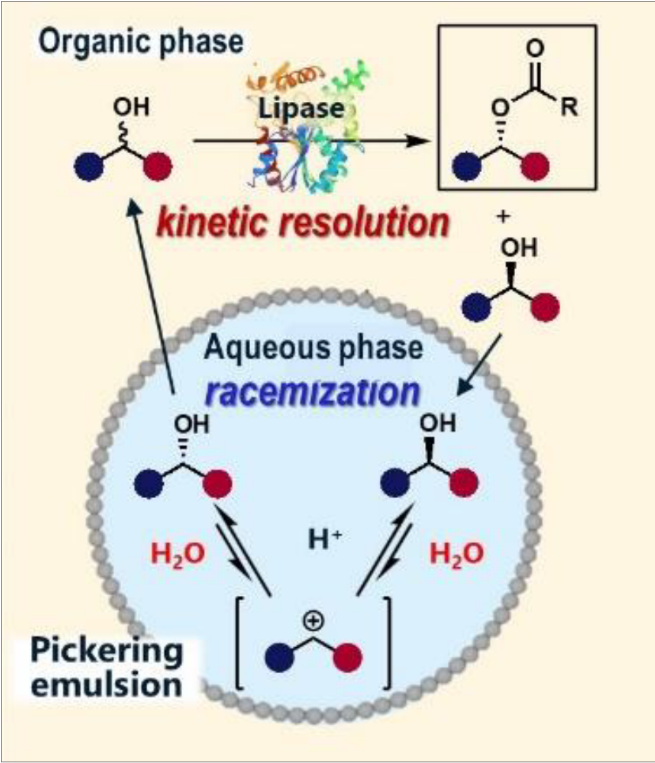
- Lipase/H2SO4 co-catalyzed DKR in Pickering emulsion (Fig. 1)[3]
- Enantiodivergent DKR for preparation of both enantiomers[4]
- DKR of 2-hydroxybiaryls without using racemization catalyst
- Kyohei Kanomata and Shuji Akai, Chemoenzymatic Dynamic Kinetic Resolution of Alcohols, In Science of Synthesis: Dynamic Kinetic Resolution (DKR) and Dynamic Kinetic Asymmetric Transformations (DYKAT), Jan. E. Bäckvall, Ed.; Thieme: Stuttgart (2023); Vol. 1, p 181–217.
- Gamal A. I. Moustafa, Yasuhiro Oki, Shuji Akai, Angew. Chem. Int. Ed. 2018, 57, 10278–10282.
- Jihoon Moon, Takusho Kin, Karin Mizuno, Shuji Akai, Kyohei Kanomata, ChemCatChem 2023, e202300878.
- Satoshi Horino, Tomoya Nishio, Shinji Kawanishi, Shinya Oki, Koichi Nishihara, Takashi Ikawa, Kyohei Kanomata, Karla Wagner, Harald Gröger, Shuji Akai, Chem. Eur. J. 2022, 28, e202203161.